BEURER EM 44 Digital Tens Device Manual

IMPORTANT SAFETY INSTRUCTIONS
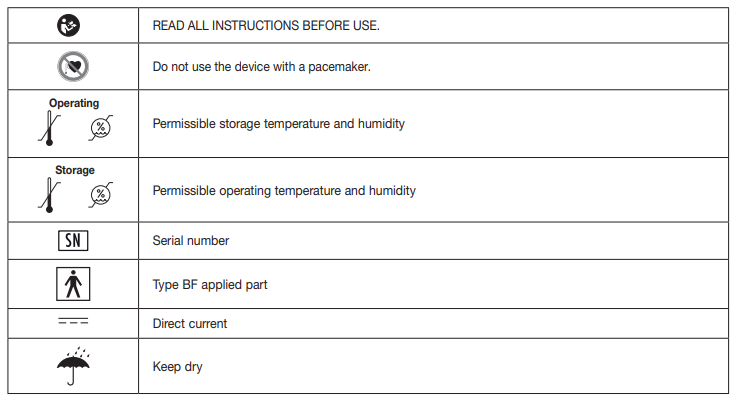
Signs and symbols
The following signs appear in the Safety Section (page 3) and in this manual on page 4, 5, 7, 8 and 15.

WARNING
Only use digital TENS units:
- On people.
- For the intended purpose and as specified in these instructions for use. Improper use can be dangerous.
- For external use.
- With the original accessories supplied, which can be re-ordered. Failure to do so invalidates the warranty.
- The long-term effects of chronic electrical stimulation are unknown.
- Do not use expired or worn gel pads which do not adhere properly. This can lead to increased current peaks and skin burns.
- Do not use stimulation over the main arteries around your neck.
- Do not use stimulation over the neck or mouth. Severe spasm of muscles may occur and the contractions may be strong enough to close the airway or cause difficulty breathing.
- Do not apply stimulation directly to the heart area across the chest or on the chest.
- Do not use on your head.
Battery Handling Safety Precautions
- Keep batteries away from children and pets. Batteries may be harmful if swallowed. Should a child or pet swallow a battery, seek
medical assistance immediately. - Use alkaline batteries.
- Use only the size and type of batteries specified.
- Be sure to follow the correct polarity when installing the batteries. Reversed batteries may cause damage to the device.
- Do not mix different types of batteries together (e.g. Alkaline and Carbon-zinc or rechargeable batteries) or old batteries with fresh ones. Always replace batteries as a simultaneous set.
- If the batteries in the device are depleted or the device will not be used for a long period of time,remove the batteries to prevent
damage or injury from possible battery leakage. - Do not try to recharge batteries not intended to be recharged; they can overheat and rupture (followbattery manufacturer’s directions.)
- Do not dispose of batteries in fire, batteries may explode or leak.
- Clean the battery contacts and also those of the device prior to battery installation.
- Remove discharged batteries from the product and dispose/recycle in compliance with all applicable laws.
Important information
Signs and symbols
The following symbols are used in these instructions for use, on the packaging and on the type plate for the device and accessories:
Introduction
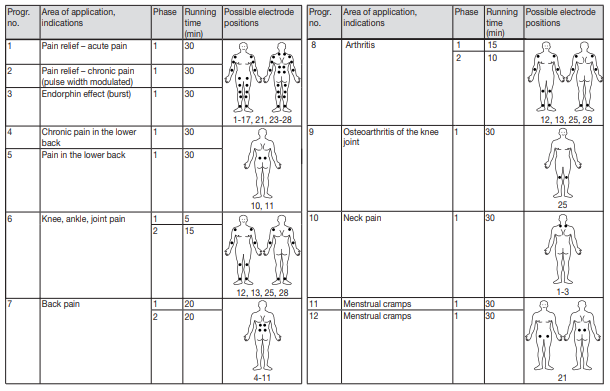
This Digital TENS Device features 15 separate programs. In all programs you can set the impulse intensity of each channel individually. You can also set various parameters in programs 13 – 15 to adjust the effect to the application area.
General Information
CAUTION
- Patients may experience skin irritation and burns beneath the stimulation electrodes applied to the skin.
- Patients may experience headache and other painful sensations during or following use.
- Patients should stop using the device and should consult with their physicians if they experience adverse reactions from the device.
Package Contents
- 1 Digital TENS Device
- 1 Belt Clip
- 2 Connection Cables
- 4 Adhesive Electrodes 1.8 x 1.8 in (45 x 45 mm)
- 3 x 1.5 V AAA Batteries
- 1 Instruction Manual
Parts and Controls
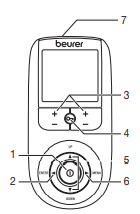
Buttons:
- ON/OFF button
- ENTER button
- Intensity setting buttons (Ch1 +/- left, Ch2 +/- right)
- Keylock
- Selection button UP and DOWN
- MENU button
- Sockets for electrodes
Electrode Pad Usage
- Do not bend or fold pads. Store pads on the plastic storage sheet when not in use.
- Do not apply ointment or any solvent to the pads or to your skin.
- The pads are pre-gelled and will adhere to your skin.
- Apply the pads only to the skin or to the plastic storage sheet provided.
- Place the pads at least two inches (5 cm) apart on your skin. The pads should never touch each other.
- Always place clean pads in accordance with illustrations provided. Make sure the components are connected properly and the pads are attached to the part of the body you wish to treat.
- Pads should not touch each other when placed onto your skin.
- Do not place on your spine or backbone.
- Pads should not touch any metal object, such as a belt buckle or necklace.
- Pads should not be placed simultaneously on the soles of both feet.
- Pads should not be placed simultaneously on the calves of both legs.
- Do not share pads with another person. This may cause a skin irritation or infection. Pads are intended for use by one person.
- Do not place or relocate the pads while the device is on. Always turn the power off before removing or changing the pad location.
- Do not leave pads attached to the skin after treatment.
- Always pull firmly on electrodes to remove them from the skin to prevent injuries in the unusual case of highly sensitive skin.
- Do not expose the device to direct sunlight or high temperatures.
Before First Use
- Remove the belt clip from the unit (if attached).
- Remove the battery compartment cover on the rear of the device by sliding it in the direction of the arrow.
- Insert three „AAA“ alkaline batteries in accordance with the polarity markings inside the compartment.
- Reattach the battery compartment cover. (Fig.1)
- Attach the belt clip, if desired.
- Attach the electrodes to the cables. (Fig.2)
- Insert the plugs of the cables into the sockets on the top of the device. (Fig.3).
- Do not pull, twist or make sharp bends in the cables. (Fig.4).
NOTE:
When the batteries are replaced or removed, all settings are restored to the factory defaults.
Positioning the Electrodes
It is important to position the electrodes properly. Consult your doctor to establish the ideal electrode positions for your intended application area. The figure on the display is intended as an initial aid to help you position the electrodes. Refer to the inside of the cover for more detailed information on how to position the electrodes.
To Use
NOTES:
- The device shuts off if it is not in use for two minutes (automatic shutoff). When the unit is switched on again, the LCD screen displays the menu selection and the most recently used menu flashes.
- A short beep sounds when a valid button is pressed. Two short beeps sound when an invalid button is pressed.
- Pause the stimulation at any time by pressing the ON/OFF button. To continue, press the ON/OFF button again and reset the desired impulse intensity.
Care and Maintenance
Adhesive Electrodes:
- To ensure that the adhesive on the electrodes lasts as long as possible, run the adhesive side under lukewarm water and pat dry with a lint-free cloth. Before cleaning with water, remove the connection cables from the electrodes.
- Reapply the electrodes to the carrier foil after treatment.
Main Unit:
- Remove the batteries from the device before cleaning.
- Clean the device after use with a soft, slightly damp cloth. You can also moisten the cloth with a mild detergent solution. Ensure that no water enters the device.
- Do not use any chemical or abrasive cleaning agents.
Storage:
- Remove the batteries from the device if you will not be using it for a prolonged period of time.
- Do not make sharp bends in the connection cables and electrodes.
- Disconnect the connection cables from the electrodes.
- Reapply the electrodes to the carrier foil after use.
- Store the device and accessories in a cool, well-ventilated space. Never place any heavy objects on the device.
Specifications
- Type… EM44
- Model… SEM 44
- Output wave form… Biphasic rectangular pulse
- Pulse length… 50–360 µs
- Pulse frequency… 1–150 Hz
- Max Output Voltage… 100 Vpp (500 ohm)
- Max Output Current… 200 mApp (500 ohm)
- Power Supply… 3 x 1.5 V AAA batteries
- Treatment Time… 5–100 minutes
- Intensity Levels… 0–50
- Operating Conditions… 50 °F–104 °F (10 °C – 40 °C), relative humidity 30–85%
- Storage Conditions… 14 °F–122 °F (-10 °C – 50 °C), relative humidity 10–95%
- Dimensions… 5.2 x 2.5 x 1.2 in (132 x 63 x 29.5 mm) including belt clip
- Weight… 3.5 oz (101 g) including belt clip
- Electrode Pad Size… 1.8 x 1.8 in (45 x 45 mm)
- Battery Life… About 15 hours
FCC Statement
Changes or modifications to the product not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment.
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and receiver.
- Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help
Warranty
Limited Lifetime Warranty For Original Purchaser
The Your Beurer Digital Tens Device Model EM 44, is warranted to be free from defects in materials and workmanship for the life of the product under normal conditions of intended use and service. This warranty extends only to the original retail purchaser and does not extend to retailers or subsequent owners. We will, at our option, repair or replace the Beurer Digital Tens Device, Model EM 44, without additional charge, for any part or parts covered by these written warranties. No refunds will be given. Repair or replacement is our only responsibility and your only remedy under this written warranty. If replacement parts for defective materials are not available, Beurer reserves the right to make product substitutions in lieu of repair or replacement.





Dear manualsclip.com admin, You always provide valuable feedback and suggestions.