Viatom BP2,Blood Pressure Monitor

The Basics
This manual contains the instructions necessary to operate the product safely and in accordance with its function and intended use. Observance of this manual is a prerequisite for proper product performance and correct operation and ensures patient and operator safety.
Safety
Warnings and Cautionary Advices
- Before using the product, please ensure that you have read this manual thoroughly and fully understand corresponding precautions and risks.
- This product has been designed for practical use, but is not a substitute for a visit to the doctor.
- This product is not designed or intended for complete diagnosis of cardiac conditions. This product should never be used as a basis for starting or modifying treatment without independent confirmation by medical examination.
- The data and results displayed on the product are for reference only and cannot be directly used for diagnostic interpretation or treatment.
- Do not attempt self-diagnosis or self-treatment based on the recording results and analysis.Self-diagnosis or self-treatment may lead to deterioration of your health.
- Users should always consult their physician if they notice changes in their health.
- We recommend not to use this product if you have a pacemaker or other implanted products.Follow the advice given by your doctor, if applicable.
- Do not use this product with a defibrillator.
- Never submerge the product in water or other liquids. Do not clean the product with acetone or other volatile solutions.
- Do not drop this product or subject it to strong impact.
- Do not place this product in pressure vessels or gas sterilization product.
- Do not disassemble and modify the product, as this could cause damage, malfunction or impede the operation of the product.
- Do not interconnect the product with other product not described in the Instruction for Use, as
this could cause damage or malfunction. - This product is not intended for use by people (including children) with restricted physical,sensory or mental skills or a lack of experience and/or a lack of knowledge, unless they are supervised by a person who has responsibility for their safety or they receive instructions from this person on how to use the product. Children should be supervised around the product to ensure they do not play with it.
- Do not allow the electrodes of the product to come into contact with other conductive parts (including earth).
- Do not use the product with persons with sensitive skin or allergies.
- DO NOT use this product on infants, toddlers, children or persons who cannot express themselves.
- Do not store the product in the following locations: locations in which the product is exposed to direct sunlight, high temperatures or levels of moisture, or heavy contamination; locations near to sources of water or fire; or locations that are subject to strong electromagnetic influences.
- This product displays changes in the heart rhythm and blood pressure etc. which may have various different causes. These may be harmless, but may also be triggered by illnesses or diseases of differing degree of severity. Please consult a medical specialist if you believe you may have an illness or disease.
- Vital signs measurements, such as those taken with this product, cannot identify all diseases.Regardless of the measurement taken using this product, you should consult your doctor immediately if you experience symptoms that could indicate acute disease.
- Do not self-diagnose or self-medicate on the basis of this product without consulting your doctor. In particular, do not start taking any new medication or change the type and/or dosage of any existing medication without prior approval.
- This product is not a substitute for a medical examination or your heart or other organ function,
or for medical electrocardiogram recordings, which require more complex measurements. - We recommend that you record the ECG curves and other measurements and provide them to
your doctor if required. - Clean the product and cuff with a dry, soft cloth or a cloth dampened with water and a neutral detergent. Never use alcohol, benzene, thinner or other harsh chemicals to clean the product or cuff.
- Avoid tightly folding the cuff or storing the hose tightly twisted for long periods, as such,treatment may shorten the life of the components.
- The product and cuff are not water-resistant. Prevent rain, sweat and water from soiling the
product and cuff. - To measure blood pressure, the arm must be squeezed by the cuff hard enough to temporarily stop blood flow through the artery. This may cause pain, numbness or a temporary red mark to the arm. This condition will appear especially when measurement is repeated successively. Any pain, numbness, or red marks will disappear with time.
- Too frequent measurements can cause injury to the patient due to blood flow interference.
- Consult your physician before using this product on an arm with an arterio -venous (A-V) shunt.
- Consult your physician before using this monitor if you have had a mastectomy or lymph node clearance.
- The pressurization of the CUFF can temporarily cause loss of function of simultaneously used monitoring product on the same limb.
- Consult your physician before using the product if you have severe blood flow problems or blood
disorders as cuff inflation can cause bruising. - Please prevent that operation of the product results in prolonged impairment of the circulation of the blood of the patient.
- Do not apply the cuff on an arm with another medical electrical equipment attached. The equipment may not function properly.
- People who have a severe circulatory deficit in the arm must consult a doctor before using the product, to avoid medical problems.
- Do not self-diagnose the measurement results and start treatment by yourself. Always consult your doctor for evaluation of the results and treatment.
- Do not apply the cuff on an arm with an unhealed wound, as this can cause further injury.
- Do not apply the cuff on an arm receiving an intravenous drip or blood transfusion. It may cause injury or accidents.
- Remove tight-fitting or thick clothing from your arm while taking a measurement.
- If the patients’ arm is outside the specified circumference range that may result in incorrect measurement results.
- The product is not intended for use with neonatal, pregnant, including pre-eclamptic, patients.
- Do not use the product where flammable gases such as anesthetic gases are present. It may cause an explosion.
- Do not use the product in the area of HF surgical equipment, MRI, or CT scanner, or in an oxygen rich environment.
- The battery intended to be changed only by service personnel with the use of a tool, and replacement by inadequately trained personnel may result in damage or burn.
- The patient is an intended operator.
- Do not carry out the servicing and maintenance while the product is in use.
- The patient can safely use all the functions of the product, and the patient can maintain the product by carefully reading Chapter 7.
- This product emits radio frequencies (RF) in the 2.4 GHz band. DO NOT use this product in locations where RF is restricted, such as on an aircraft. Turn off the Bluetooth feature in this product and remove batteries when in RF restricted areas. For further information on potential restrictions refer to documentation on the Bluetooth usage by the FCC.
- DO NOT use this product with other medical electrical (ME) equipment simultaneously. This may result in incorrect operation of the product and/ or cause an inaccurate blood pressure readings and/ or EKG recordings.
- Sources of electromagnetic disturbance may affect this product (e.g. mobile telephones,microwave cookers, diathermy, lithotripsy, electrocautery, RFID, electromagnetic anti-theft systems, and metal detectors), please try to stay away from them when making measurements.
- The use of accessories and cables other than those specified or provided by manufacture could result in increased electromagnetic emission or decreased electromagnetic immunity of the product and result in improper operation.
- Interpretations made by this product are potential findings, not a complete diagnosis of cardiac conditions. All interpretations should be reviewed by a medical professional for clinical decision-making.
- DO NOT use this product in the presence of flammable anesthetics or drugs.
- DO NOT use this product while charging.
- Remain still while recording an ECG.
The detectors of ECG have been developed and tested on Lead I and II recordings only.
Introduction
Intended Use
The device is indented to measure blood pressure or electrocardiogram (ECG) in home or healthcare facilities environment.The device is a blood pressure monitor intended for use in measuring blood pressure and pulse rate in
the adult population. The product is intended to measure, display, store and review adults’ single-channel ECG rhythms and gives some suggested symptoms such as regular beat, irregular beat, low HR and high HR.
Contraindications
This product is contraindicated for use in ambulatory environments.This product is contraindicated for use on aircraft.
About the product
name: Blood Pressure Monitor
Product model: BP2 (include NIBP+ECG), BP2A (only NIBP)
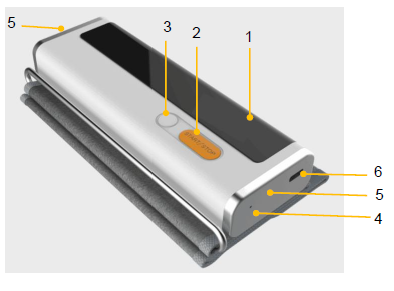
LED screen
- Display date, time and power status, etc.
- Display ECG and blood pressure measurement process and results.
Start/ Stop button
- Power On/ Off
- Power On: Press the button to power on.
- Power off: Press and hold the button to power off.
- Press to power on the product and press again to start measuring blood pressure.
- Press to power on the product and touch the electrodes to start measuring ECG.
Memory button
Press to review historical data.
LED indicator
- Blue light is on: the battery is being charged.
- The blue light is off: the battery is fully charged not charging
ECG electrode
Touch them to start measuring ECG with different methods.
USB connector
It connects with a charging cable.
Symbols
| Symbol | Meaning |
 | Application part type BF |
 | Manufacturer |
| CE0197 | In conformity with Directive 93/42/EEC |
 | European Representative |
 | Symbol for “ENVIRONMENT PROTECTION – Waste electrical products should not be disposed of with household waste. Please recycle where facilities exist. Check with your local authority or retailer for recycling advice”. |
| IP22 | Against ingress of solid foreign objects ≥12.5mm diameter; Against dripping (15° tilted) |
 | Follow operating instructions |
Using the Product
Charge the Battery
Use the USB cable to charge the product. Connect the USB cable to a USB charger or to the PC. A full charge will need 2 hours. When the battery is charged fully the indicator will be blue. The product works with very low power consumption and one charge usually works for months. On-screen battery symbols which indicate the battery status can be seen on the screen.
Note: The product cannot be used during charging, and if choosing a third-party charging adaptor, select one that complies with IEC60950 or IEC60601-1.
Measure Blood Pressure
Applying the arm cuff
- Wrap the cuff around the upper arm, about 1 to 2 cm above the inside of the elbow, as shown.
- Place the cuff directly against the skin, as clothing may cause a faint pulse and result in a measurement error.
- Constriction of the upper arm, caused by rolling up a shirtsleeve, may prevent accurate readings.
- Confirm that the artery position mark is lined up with the artery.
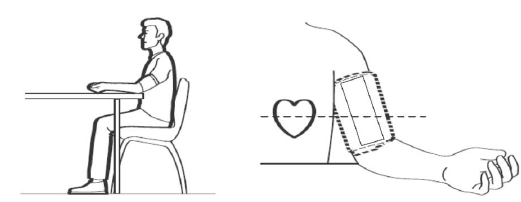
How to sit correctly
To take a measurement, you need to be relaxed and comfortably seated. Sit in a chair with your legs uncrossed and your feet flat on the floor. Place your left arm on a table so the cuff is level with your heart.
Troubleshooting
| Problem | Possible Cause | Recommended Action |
|
The product cannot pair. | The product is out of Bluetooth range. | Verify product is in Bluetooth range while being paired (approximately 1.5 meters). |
| The radio frequency interference (ex. microwave) | Conduct the pairing process away from possible interference source. | |
| The product doesn’t respond to the button press. | The battery may be low. | Charge battery and try again. |
| The product is running in an unexpected status. | Reset the product by press and hold the button for 8s. | |
| The device might be damaged. | Please contact your local distributor. | |
| Cannot get blood pressure measurement results. | The measurement is interrupted by arm movement or unexpected bulb squeeze. | Keep arm still and don’t squeeze the bulb during deflating-measure phase. |
| There is an over-leakage of Press. | Check if the hose connection is loose. | |
|
Cannot get ECG-measured results. |
Hold the electrode is too loose. | Please hold the electrode properly, and ensure no skin contact between the right and left hand. |
| The skin of the hand is too dry. | Please keep the skin of hand a little moist. |
Accessories
| Model | Description |
| 540-00240-00 | MICRO USB charge cable |
Specifications
| Classifications | ||
| Degree of protection against electrical shock | Type BF | |
| Protection against electric shock | Internally powered equipment | |
| Electro-magnetic Compatibility | Group I, Class B | |
| Apply part | Cuff and ECG electrode | |
| Environmental | ||
| Item | Operating | Storage |
| Temperature | 5°C to 45°C | -25°C to 70°C |
| Relative humidity (non-condensing) | 10% to 95% | 10% to 95% |
| Barometric | 700hPa to 1060 hPa | 700hPa to 1060 hPa |
| Degree of dust & water resistance | IP22 | |
| Drop test | 1.0 m | |
| Physical | ||
| Size | 135 mm×46mm×21mm (main unit) | |
| Weight | about 220 g (main unit) | |
| Cuff size | 22-42 cm | |
| Wireless connectivity | Built-in Bluetooth 4.0 BLE | |
| Power Supply | ||
| Charge input | Micro USB, DC5V | |
| Battery type | Rechargeable lithium-polymer battery | |
| Charge time | 2 hours | |
| Blood Pressure | ||
| Technology | Oscillometric method | |
| Pressure measurement range | 0 – 300 mmHg | |
| Pressure measurement accuracy | ±3mmHg or 2%, whichever is greater. | |
| Pulse rate range | 40 to 200 /min | |
| Pulse rate accuracy | ±2 /min | |
| Clinical accuracy | Meet ISO 81060-2:2013 | |
| ECG | ||
| Lead type | Integrated ECG electrodes | |
| ECG channel | Single Channel | |
| Measure mode | Continuous | |
| Input impendence | ≥10MΩ, 10Hz | |
| CMRR | >60 dB |
| Center Frequency | 50/60 Hz |
| Linearity and dynamic range | 10mV (Peak-to-valley) |
| Frequency response | 0.67 to 40 Hz |
| Gain error (Max.) | ±10% |
| HR measurement range | 30 to 250 /min |
| Accuracy | ±2 /min or ±2%, whichever is greater. |
| Memory | |
| Capacity | BP2: up to 100 readings for Blood Pressure data + 10 records for ECG data. BP2A: up to 100 readings for Blood Pressure data |
| Bluetooth RF | |
| Frequency range | 2.402-2.480 GHz GFSK Modulation Adaptive Frequency Hopping (AFH) |
|
Wireless Quality of Service (QoS) | Transmission Distance: 1.5m Transmission Time: ≤0.5s for one Blood Pressure reading ≤10s for one ECG record Data integrity: 100% |
| Network topology | Point-to-Point |
| Bandwidth | 1Mbps |
| Durable period | |
| Expected service life | 3 years |
Maintenance and Cleaning
Maintenance
To protect your product from damage, please observe the following:
- Store the product and the components in a clean, safe location.
- Do not wash the product and any components or immerse them in water.
- Do not disassemble or attempt to repair the product or components.
- Do not expose the product to extreme temperatures, humidity, dust or direct sunlight.
- The cuff contains a sensitive air-tight bubble. Handle this carefully and avoid all types of straining through twisting or buckling.
- Clean the product with a soft, dry cloth. Do not use petrol, thinners or similar solvent. Spots on the cuff can be removed carefully with a damp cloth and soapsuds. The cuff must not be washed!
FCC Statement
FCC ID: 2ADXK-8621
Any Changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment. This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
- This device may not cause harmful interference, and
- this device must accept any interference received, including interference that may cause undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device,pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and the receiver.
- Connect the equipment to an outlet on a circuit different from that to which the receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help.
Electromagnetic Compatibility
The product meets the requirements of EN 60601-1-2.
Warnings and Cautionary Advice
- Using accessories other than those specified in this manual may result in increased electromagnetic emission or decreased electromagnetic immunity of the equipment.
- The product or its components should not be used adjacent to or stacked with other equipment.
- The product needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided below.
- Other products may interfere with this product even though they meet the requirements of CISPR.
- When the inputted signal is below the minimum amplitude provided in technical specifications, erroneous measurements could result.
- Portable and mobile communication equipment may affect the performance of this product.
- Other products that have RF transmitters or sources may affect this product (e.g. cell phones, PDAs, and PCs with wireless function).
| Guidance and Declaration – Electromagnetic Emissions | ||
| The Blood Pressure Monitor is intended for use in the electromagnetic environment specified below. The customer or the user of the Blood Pressure Monitor should assure that it is used in such an environment. | ||
| Emission tests | Compliance | Electromagnetic environment – guidance |
| RF emissions CISPR 11 | Group 1 | The Blood Pressure Monitor uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. |
| RF emissions CISPR 11 | Class B | The Blood Pressure Monitor is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. |
| Harmonic emissions IEC61000-3-2 | N/A | |
| Voltage Fluctuations / Flicker Emissions IEC 61000-3-3 | N/A | |
| The Blood Pressure Monitor is intended for use in the electromagnetic environment specified below. The customer or the user of the Blood Pressure Monitor should assure that it is used in such an environment. | |||
| Immunity test | IEC60601 test level | Compliance level | Electromagnetic environment – guidance |
| Electrostatic discharge (ESD) IEC 61000-4-2 | ± 8kV contact
±2kV, ±4kV, ±8kV, ±15kV air | ± 8kV contact
±2kV, ±4kV, ±8kV, ±15kV air | Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. |
| Electrical fast transient/ burst IEC 61000-4-4 | ± 2kV for power supply lines 100kHz repetition frequency ± 1kV for input/output lines |
N/A |
— |
| Surge IEC 61000-4-5 | ± 0.5kV, ±1kV differential mode line-line | N/A | — |
|
Voltage dips, short Interruptions and Voltage variations on power supply input lines IEC 61000-4-11 | 0% UT (100% dip in UT) for 0.5 cycles at 0°, 45°, 90°, 135°,180°, 225°, 270°, and 315°
0% UT (100% dip in UT) for 1 cycle at 0°
70% UT (30% dip in UT) for 25/30 cycles at 0°
0% UT (100% dip in UT) for 250/300 cycles at 0° |
N/A |
— |
| Power frequency (50/60 HZ) magnetic field IEC 61000-4-8 |
30 A/m, 50/60Hz |
30 A/m, 50/60Hz | Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. |
| Note: UT is the AC mains voltage prior to application of the test level. | |||





